The new phiDB lite platform will make PROMs accessible, operationalizing within 2 weeks.
SAN FRANCISCO, Calif. (June 8, 2016) – Ortech Systems launched a streamlined patient-reported outcomes platform, phiDB lite, to help orthopaedic practices begin collecting patient-reported outcomes (PROMs) within two weeks. This will allow any practice to compare providers, to share a summary with patients in order to show their progress, and to include PROMs as part of bundled payment reports. The offering launches officially at the American Association for Orthopaedic Executives (AAOE) conference in San Francisco, where Ortech is exhibiting at booth #211.
The creation of Ortech’s phiDB lite offering follows the AAOS Board of Directors’ approved recommendation for PROMs across specialties in February. A Quality Outcomes Data Work Group for AAOS convened in 2015 to evaluate data collection tools. Review the recommendation by specialty area here from AAOS.
“Deciding what to measure used to be a hurdle for orthopaedic practices. Now that AAOS has provided guidelines on what to measure, orthopaedic practices are assured their data will support quality improvement,” said Michael Barr, Vice President of Ortech Systems. “This was Ortech’s cue to streamline PROMs collection, so any practice can operationalize quickly.”
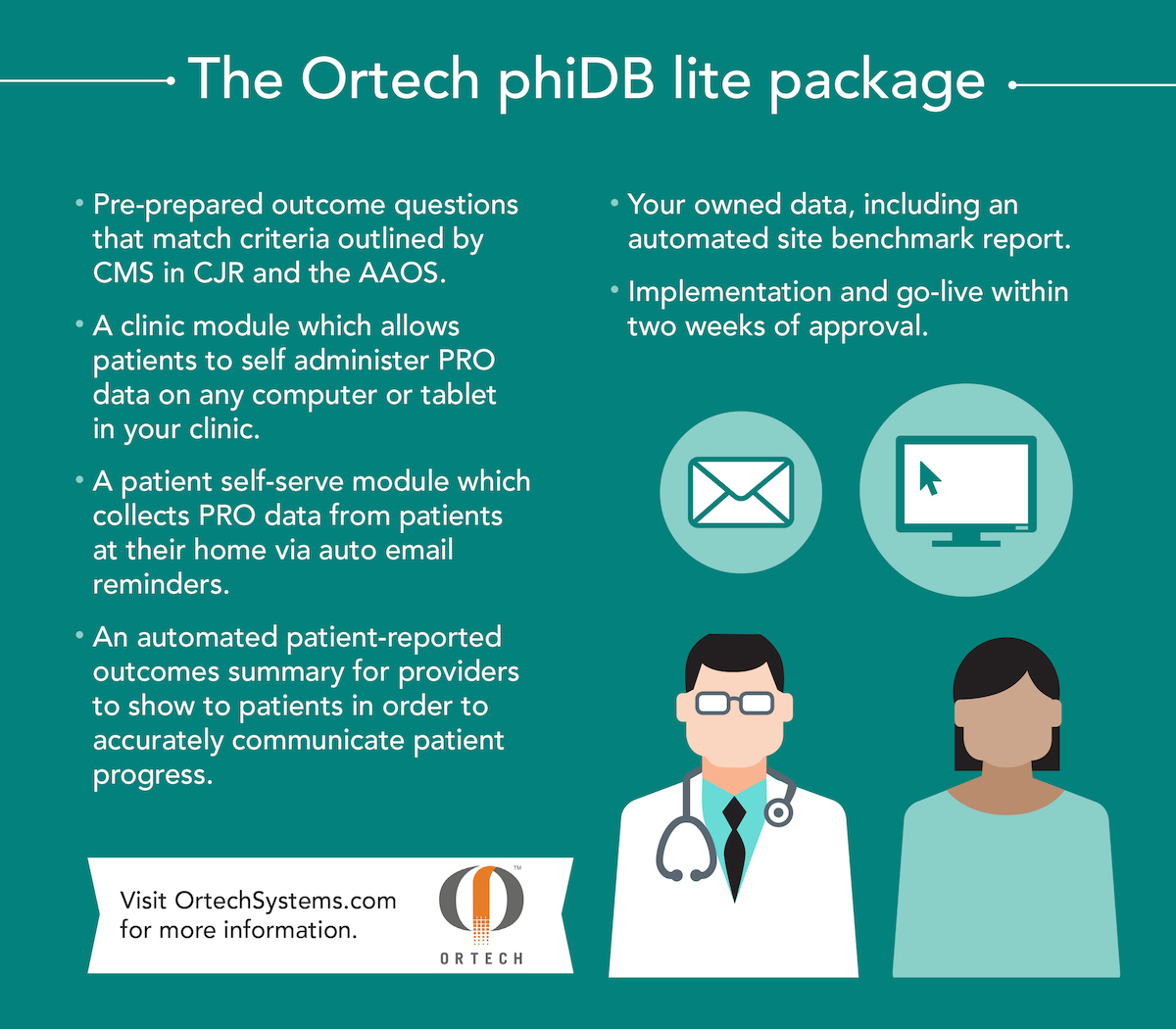
The phiDB Lite package provides –
- Pre-prepared outcome questions that match criteria recommended by AAOS across any orthopaedic specialty area, and outlined by CMS in CJR
- A web-based clinic module which captures PRO data on any computer or tablet in your clinic
- A patient self-serve module which collects PRO data from patients at their home via auto-sent email links and reminders
- An automated patient-reported outcomes status report for providers to share with patients and communicate progress
- Your owned data, including automated site benchmark reports
- Implementation and go-live within two weeks of approval
The dataset is aligned with AAOS recommendations, which include –
- Quality of life and
- Two Orthopaedic Sub-Specialty Surveys, which represent: Foot and Ankle, Knee (ACL), Knee (Osteoarthritis), Hip (Osteoarthritis), Shoulder, Shoulder (Instability), Elbow, Wrist, Hand, Spine
Ortech provides comprehensive data collection to support evidence-based practices for national and state-wide registries, health systems and orthopaedic clinics. The phiDB lite product supports an extension to large and small practices that focuses on PROMs exclusively. Compare Ortech’s services here.
To schedule a demo, or request a sample benchmarking report, please contact Michael Barr at michael.barr@ortechsystems.com or 1-888-678-3240 ext. 104. View Ortech’s graphic on optimizing patient compliance and introducing PROMs in the workflow here.
###
About Ortech Systems
Ortech guides specialty clinics, health systems and state-wide registries in achieving evidence-based orthopaedics with its complete data-capture platform. Organizations have the ability to mine data – connecting patient to implant to clinical outcome to patient-reported outcome. Ortech has designed and currently manages large-scale registries including the California Joint Replacement Registry, the Michigan Arthroplasty Replacement Collaborative Quality Initiative and the North American Spine Society Registry.
Ortech`s web-based product was designed in collaboration with leading orthopaedic surgeons across North America, allowing healthcare providers to efficiently capture intra-operative and implant data, as well as patient-reported outcomes in order to measure and analyze patient progress. Many hospitals and private clinics use this data to measure quality of care, track and improve the patient experience and benchmark against national standards.